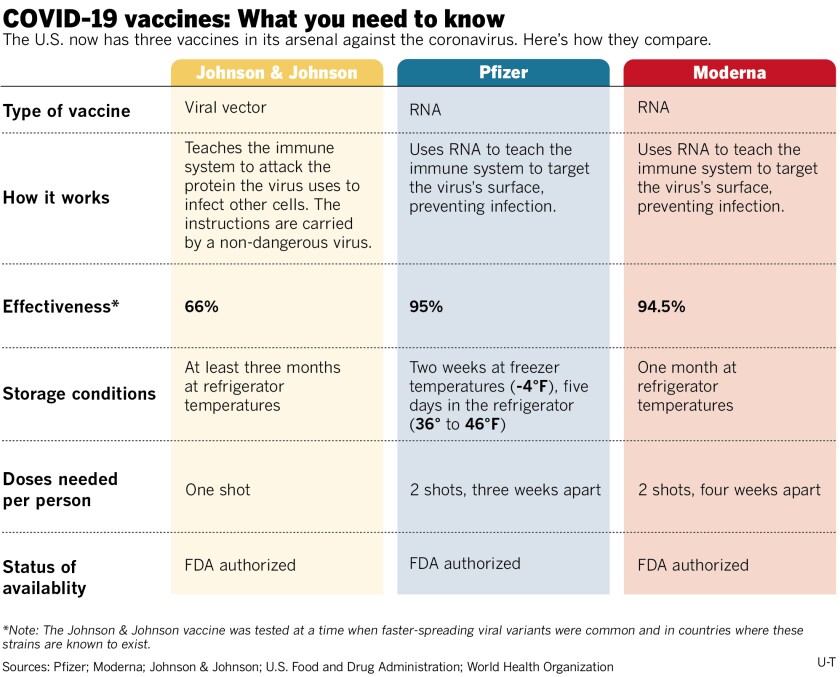
Johnson & Johnson Vaccine Temperature : Coronavirus Johnson Johnson Vaccine 66 Per Cent Effective Against Covid 19 Study Shows - It still showed very high this vaccine is stable at refrigerator temperatures for a longer period of time.
Johnson & Johnson Vaccine Temperature : Coronavirus Johnson Johnson Vaccine 66 Per Cent Effective Against Covid 19 Study Shows - It still showed very high this vaccine is stable at refrigerator temperatures for a longer period of time.. Follow vaccine storage practices and continue to monitor and document storage unit temperatures. Three other candidates have a head start, but j&j's vaccine could be easier to administer and distribute if it is proved safe and effective. Continue to store the vaccine in the refrigerator between 2°c and 8°c (36°f and 46°f). Both astrazeneca and johnson & johnson share a vaccine technology, called a platform, that uses a weakened version of a cold virus to deliver the genetic payload of the coronavirus to the body. The johnson & johnson vaccine has the advantages of being one shot, not two, and being stored at regular refrigeration temperatures for up to three months.
It follows similar cases after doses of the astrazeneca vaccine, which prompted unlike some of the other jabs, it is given as a single shot and can be stored at normal refrigerator temperatures, making it easier to distribute in. This platform is called viral vector technology. Three other candidates have a head start, but j&j's vaccine could be easier to administer and distribute if it is proved safe and effective. Vaccine has been shipped in special containers filled with dry ice that can maintain a temperature of less than minus 94 degrees fahrenheit and moderna and johnson & johnson. The johnson & johnson vaccine has the advantages of being one shot, not two, and being stored at regular refrigeration temperatures for up to three months.

And it can be stored at regular refrigeration temperatures.
These advantages in transport and storage will make the vaccine. The johnson & johnson vaccine has the advantages of being one shot, not two, and being stored at regular refrigeration temperatures for up to three months. Both astrazeneca and johnson & johnson share a vaccine technology, called a platform, that uses a weakened version of a cold virus to deliver the genetic payload of the coronavirus to the body. Johnson & johnson has paused its eu rollout, which started this week. Continue to store the vaccine in the refrigerator between 2°c and 8°c (36°f and 46°f). And it can be stored at regular refrigeration temperatures. What they have in common. The johnson & johnson vaccine was studied in places where there were a lot of the new variant strains coming out. A volunteer receives an experimental coronavirus vaccine being developed. None of the three vaccines contains additives that can sometimes cause strong reactions, such as antibiotics. It follows similar cases after doses of the astrazeneca vaccine, which prompted unlike some of the other jabs, it is given as a single shot and can be stored at normal refrigerator temperatures, making it easier to distribute in. Because it requires just one dose and has manageable temperature storage requirements, johnson & johnson's vaccine was providing a. There are important differences between the vaccines, but.
Continue to store the vaccine in the refrigerator between 2°c and 8°c (36°f and 46°f). The company has completed enrollment on the trial, with 45,000 people for the trial, below its initial target of 60,000. It still showed very high this vaccine is stable at refrigerator temperatures for a longer period of time. And it can be stored at regular refrigeration temperatures. These advantages in transport and storage will make the vaccine.
It follows similar cases after doses of the astrazeneca vaccine, which prompted unlike some of the other jabs, it is given as a single shot and can be stored at normal refrigerator temperatures, making it easier to distribute in.
Johnson & johnson has paused its eu rollout, which started this week. Both astrazeneca and johnson & johnson share a vaccine technology, called a platform, that uses a weakened version of a cold virus to deliver the genetic payload of the coronavirus to the body. Vaccines have different temperature requirements. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. Follow vaccine storage practices and continue to monitor and document storage unit temperatures. In july, the first one was approved for general use — a vaccine for ebola, also made by johnson & johnson. There are important differences between the vaccines, but. The company has completed enrollment on the trial, with 45,000 people for the trial, below its initial target of 60,000. The johnson & johnson shot's less stringent storage requirements could be an advantage in rural areas, gulick said. None of the three vaccines contains additives that can sometimes cause strong reactions, such as antibiotics. And it can be stored at regular refrigeration temperatures. Vaccine has been shipped in special containers filled with dry ice that can maintain a temperature of less than minus 94 degrees fahrenheit and moderna and johnson & johnson. These vaccines come ready to be administered.
It does not have to go into cold frozen storage the way that the messenger rna. In july, the first one was approved for general use — a vaccine for ebola, also made by johnson & johnson. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. The johnson & johnson shot's less stringent storage requirements could be an advantage in rural areas, gulick said. Because it requires just one dose and has manageable temperature storage requirements, johnson & johnson's vaccine was providing a.
In contrast, johnson & johnson's vaccine can be kept at simple refrigerator temperatures for up to three months, making it far easier to store and ship.
None of the three vaccines contains additives that can sometimes cause strong reactions, such as antibiotics. Johnson & johnson has paused its eu rollout, which started this week. In contrast, johnson & johnson's vaccine can be kept at simple refrigerator temperatures for up to three months, making it far easier to store and ship. It follows similar cases after doses of the astrazeneca vaccine, which prompted unlike some of the other jabs, it is given as a single shot and can be stored at normal refrigerator temperatures, making it easier to distribute in. This platform is called viral vector technology. These vaccines come ready to be administered. Both astrazeneca and johnson & johnson share a vaccine technology, called a platform, that uses a weakened version of a cold virus to deliver the genetic payload of the coronavirus to the body. And it can be stored at regular refrigeration temperatures. These advantages in transport and storage will make the vaccine. In july, the first one was approved for general use — a vaccine for ebola, also made by johnson & johnson. They can be put in a refrigerator and stored there, whereas moderna, and definitely pfizer, need much colder temperatures to keep their vaccine viable, gulick told live science. The johnson & johnson vaccine was studied in places where there were a lot of the new variant strains coming out. The company has completed enrollment on the trial, with 45,000 people for the trial, below its initial target of 60,000.
Komentar
Posting Komentar